Irrigation Water Quality for Turfgrass Sites

Irrigation water quality, formerly a minor concern in the eastern United States, is becoming an important issue for managers of golf courses, athletic fields, and institutional grounds in Pennsylvania. With the demand for potable water increasing, pressure is being placed on users of irrigation water to considering using non-potable alternatives, such as recycled water. Because water quality can influence soil quality and turfgrass performance, it is advisable to test irrigation water periodically for factors that can compromise the turf/soil system. Listed in the following paragraphs are some suggested guidelines to follow when interpreting results of irrigation water analyses.
pH
Irrigation water can be classified as acid, neutral, or alkaline. The degree of acidity or alkalinity of water can be described by a pH value. pH values range from 0 to 14; any value below 7.0 is considered acid, a value of 7.0 is neutral, and a pH above 7 is alkaline. Thus, water with a pH of 5.8 is acidic, whereas water with a pH of 7.9 is alkaline.
Water becomes more acidic as pH values below 7.0 decrease numerically. In fact, there is a ten-fold increase in acidity for every decrease by one whole pH unit. For example, water with a pH of 5.5 is ten times more acid than water with a pH of 6.5; and water with pH of 4.5 is 100 times more acid than water with a pH of 6.5. Similarly, for every whole unit increase in pH above 7, there is a ten-fold increase in alkalinity. pH values are usually reported to the nearest 1/10th of a whole unit on an irrigation water test report (ie. 6.2, 7.0, 8.5).
The pH of irrigation water should be determined in a laboratory and reported as part of your test report. Water with a pH in the range of 6.0 to 7.0 is most desirable for use on turfgrasses. Water with pH values outside of this range may not directly influence turfgrass performance, but indicates a need to evaluate other chemical components of the water.
Bicarbonates and carbonates
Bicarbonate (HCO3–) and carbonate (CO3-2) are common constituents of irrigation water, and can influence soil properties and turfgrass performance. If bicarbonate and/or carbonate levels are high (>120 and 15 mg/L, respectively), these ions can react with calcium and magnesium in the soil to form insoluble calcium carbonate and magnesium carbonate (lime). This reaction reduces the amount of free calcium and magnesium in soil, allowing sodium to compete for and occupy negatively-charged exchange sites on clay particles. Excess sodium in clay results in destruction of soil structure and reduced water percolation though the soil profile. This effect is referred to as the sodium permeability hazard.
Residual Sodium Carbonate (RSC)
The sodium permeability hazard for irrigation water is usually assessed when bicarbonate and carbonate levels are >120 and 15 mg/L, respectively. Residual sodium carbonate (RSC) is a common means of assessing the sodium permeability hazard, and takes into account the bicarbonate/carbonate “and” calcium/magnesium concentrations in irrigation water. RSC is important because it’s not the absolute bicarbonate and carbonate concentrations that are important, but instead, the relative concentrations of bicarbonate and carbonate compared to concentrations of calcium, magnesium, and sodium.
RSC is calculated as follows:
RSC (meq/L) = (HCO3– + CO3-2) – (Ca + Mg)
Note that for this equation, all concentrations are expressed in meq/L. Typically, water with a RSC value of 1.25 meq/L or lower is safe for irrigating turf. RSC values between 1.25 and 2.5 meq/L is marginal, and above 2.5 meq/L is considered excessive.
Electrical conductivity (EC) and total dissolved solids (TDS)
EC is a measure of the degree in which water conducts electricity. It is determined by passing an electrical current through a water sample and recording the resistance in mmhos/cm or dS/m. EC is used to estimate the concentration of TDS in water, using the following equation:
TDS (ppm or mg/L) = EC (mmhos/cm or dS/m) × 640
TDS is occasionally referred to as total dissolved salts (also abbreviated TDS) or total soluble salts (TSS), and both are determined using the same equation.
Acceptable TDS concentrations for turfgrass irrigation range from 200 to 500 mg/L (EC = 0.31 to 0.78 mmhos/cm). TDS concentrations higher than 2,000 mg/L (EC = 3.1 mmhos/cm) can damage turfgrasses. If using irrigation water with a TDS concentration higher than 500 mg/L, attention should focus on irrigation duration and frequency, drainage, and turfgrass species selection.
Sodium
Sodium exists in nearly all irrigation water and is not necessarily a cause for concern unless high concentrations are present. High concentrations (> 70 mg/L) can be detrimental to both turf and soils. Sodium in irrigation water can be absorbed by roots and foliage, and foliar burning can occur if sufficient amounts accumulate in leaf tissue. Grasses grown on golf course putting greens (creeping bentgrass and annual bluegrass) are particularly susceptible to sodium toxicity because they are mowed very short, irrigated frequently, and subjected to other stresses.
Sodium absorption ratio (SAR)
The relative concentrations of sodium, calcium, and magnesium are important determinants of irrigation water quality. Calcium and magnesium play a major role in maintaining structure of clay-containing soils. If water with excess sodium and low calcium and magnesium is applied frequently to clay soils, the sodium will tend to displace calcium and magnesium on clay particles, resulting in breakdown of structure, precipitation of organic matter, and reduced permeability.
SAR is used to assess the relative concentrations of sodium, calcium, and magnesium in irrigation water and provide a useful indicator of its potential damaging effects on soil structure and permeability.
Typically a SAR value below 3.0 is considered very safe for turfgrasses. Over time, water with a SAR of 9.0 or above can cause significant structural damage to clay soils. Sandy soils are not as susceptible to structure and permeability problems, and can tolerate higher SAR values (up to 10 in most cases).
Chloride
Chloride contributes to salinity of irrigation water, and when concentrations are high enough, can be toxic to plants. Turfgrasses are not particularly sensitive to chloride, and can tolerate levels up to 100 mg/L. Turfgrasses can sustain injury when irrigated with water containing >355 mg/L of chloride. Turfgrass managers should be aware that some ornamental plants are sensitive to chloride concentrations above 70 mg/L.
Boron
Boron is essential for plant growth at very low concentrations. However, it can be quite toxic to some ornamental plants at concentrations as low as 1 to 2 mg/L in irrigation water; with symptoms appearing as necrosis on margins of older leaves. By comparison, turfgrasses are more tolerant of boron, and are capable of growing in soils with boron concentrations of 10 mg/Kg. To be safe, it is best to use irrigation water with boron concentrations < 2 mg/L for turfgrasses.
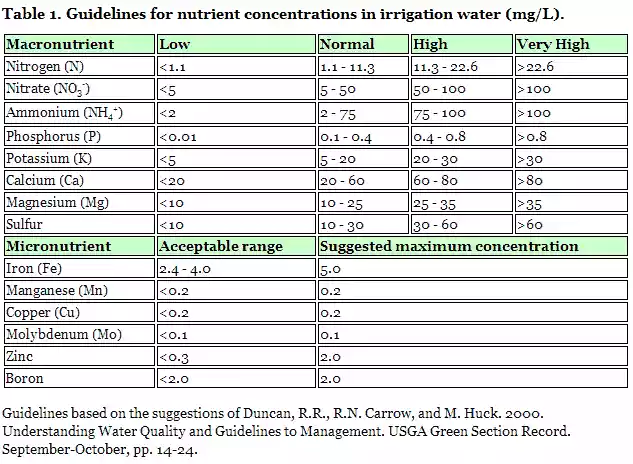
Nutrients in irrigation water
Irrigation water contains plant nutrients in varying concentrations. Depending on concentrations, nutrients can influence fertility programs and have an environmental impact on ground and surface water. Nitrogen has a significant influence on plant growth, and may present a hazard for drinking water sources if nitrate levels are 10 mg/L or more. Phosphorus concentrations should be as low as possible (lower than 1.0 mg/L) to avoid causing algal blooms in holding ponds and phosphorus loading in surface streams and lakes. Guidelines for nutrient concentrations are provided in Table 1.